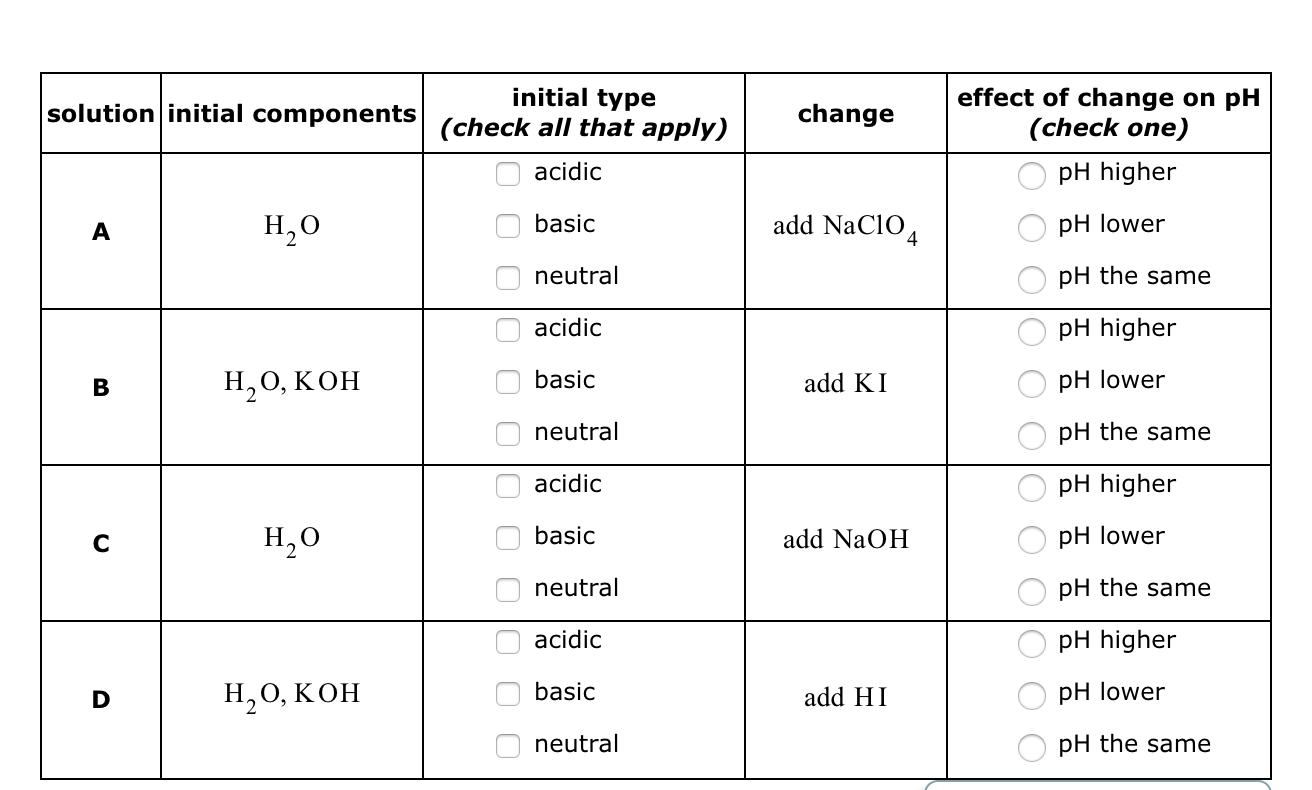
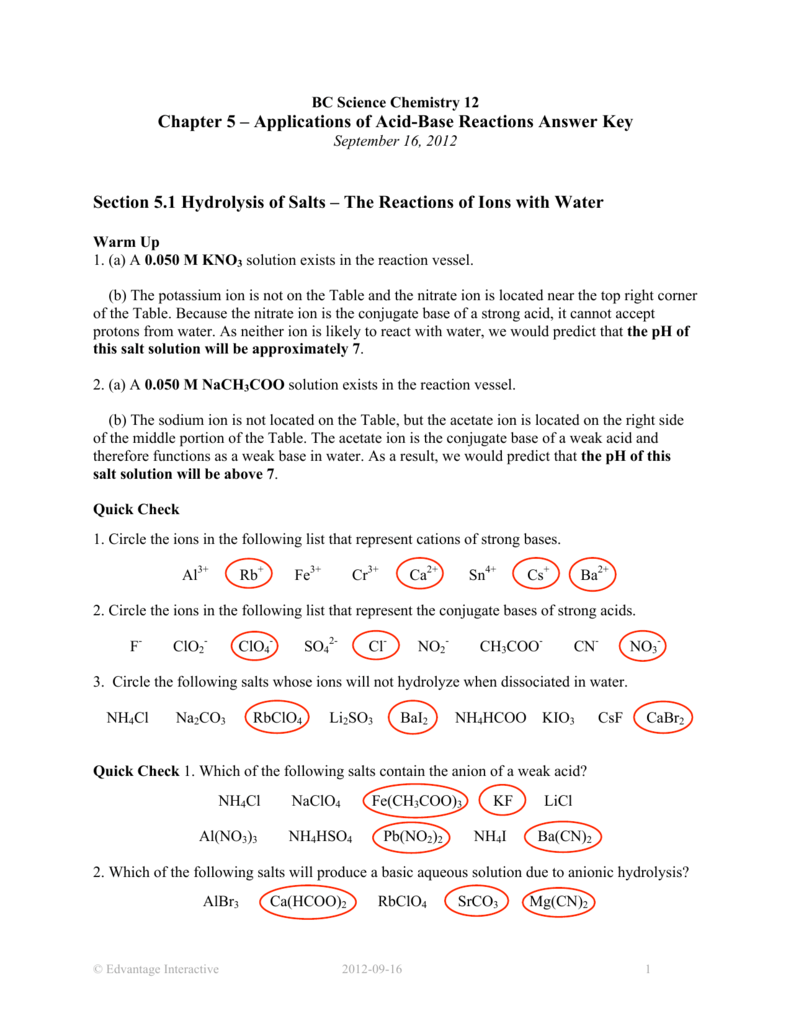
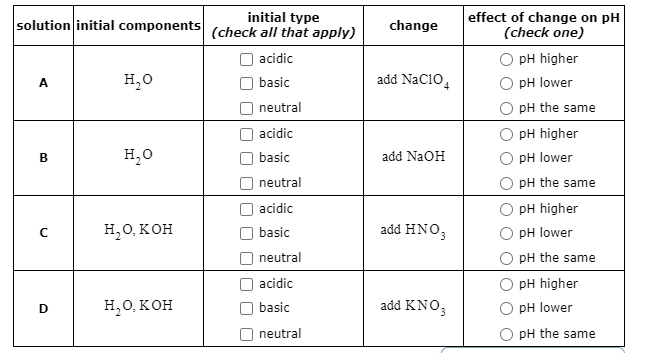
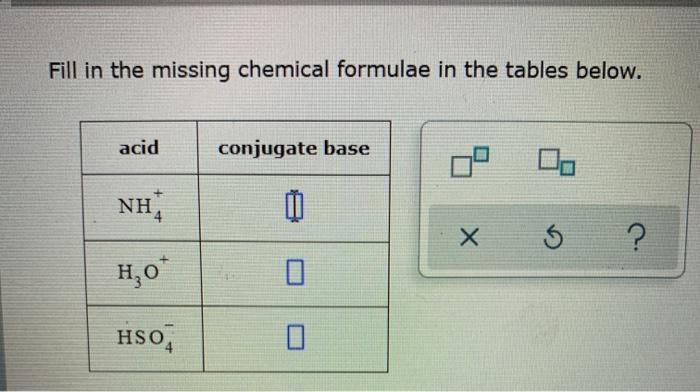
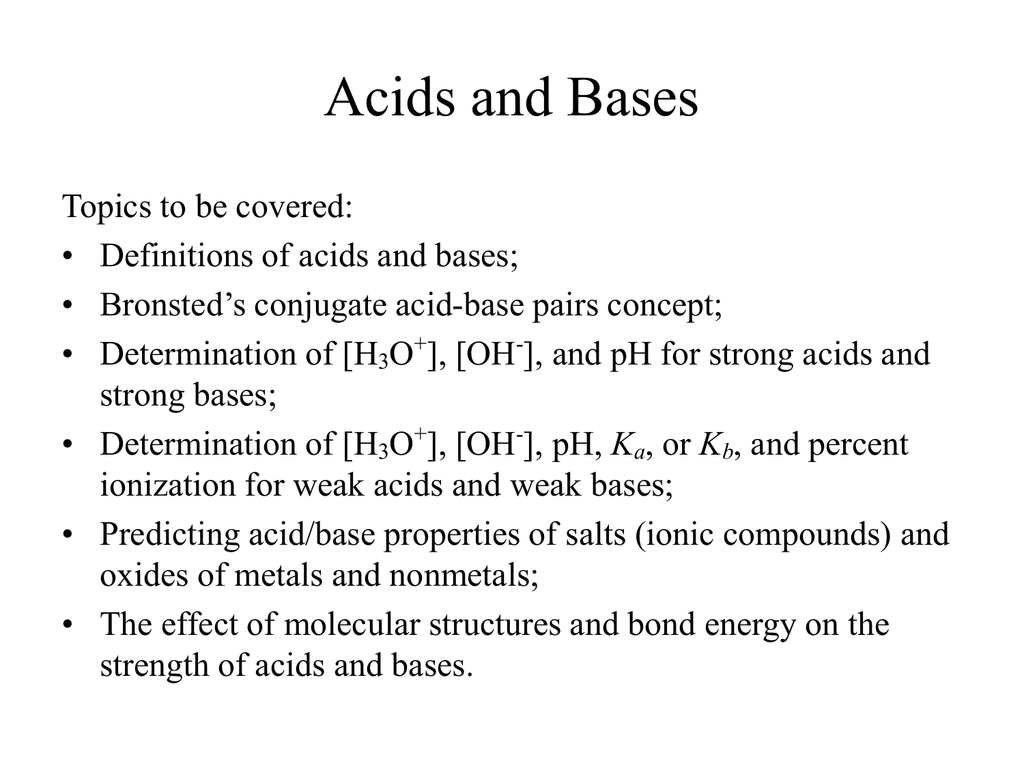
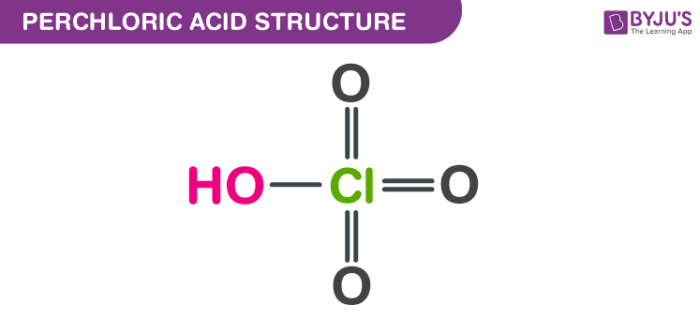
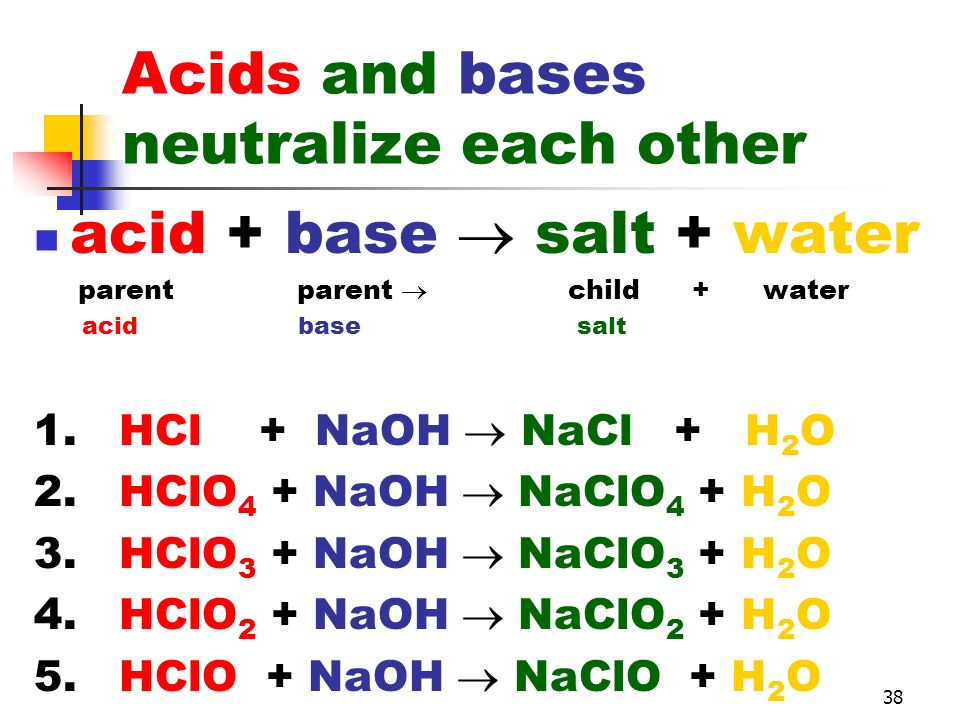
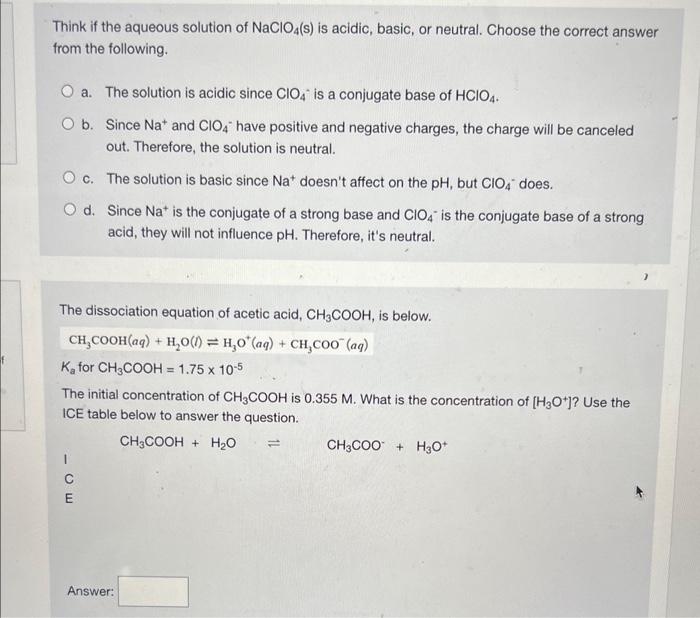
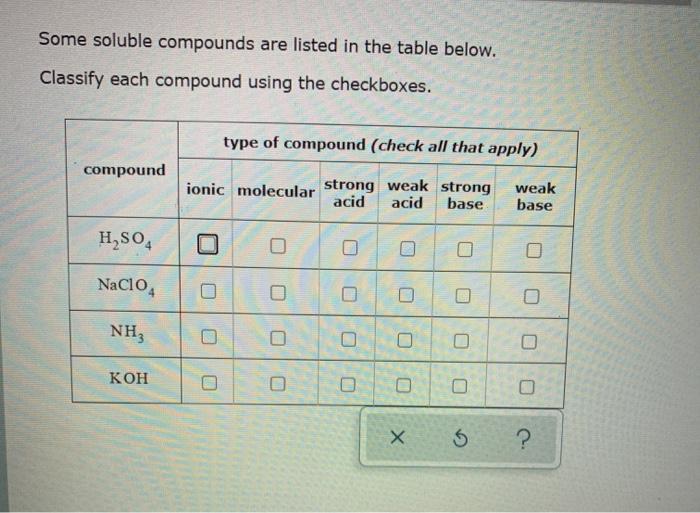
Predict if the solutions of the following salts are neutral, acidic or basic. NaCl, KBr, NaCN, NH4NO3,NaNO2 and KF

Solid Acid/Base Catalysis in Sub- and Supercritical Water | Industrial & Engineering Chemistry Research
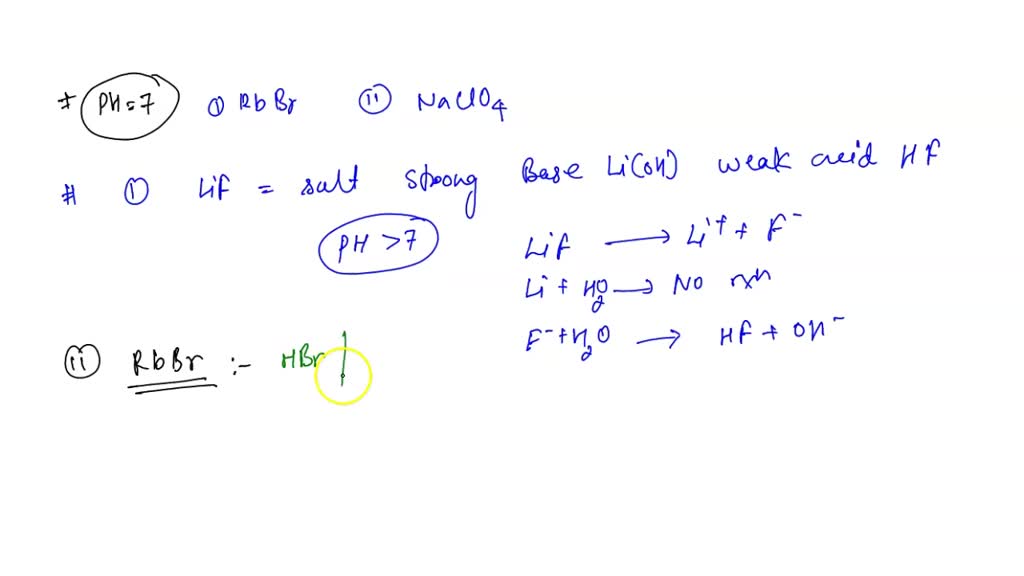
SOLVED: I have a theoretical question about: 9) Which of the following 0.5 M aqueous salt solutions will have a pH of 7.0 at LiF RbBr NaClO4 NH4Cl A) LiF only B)
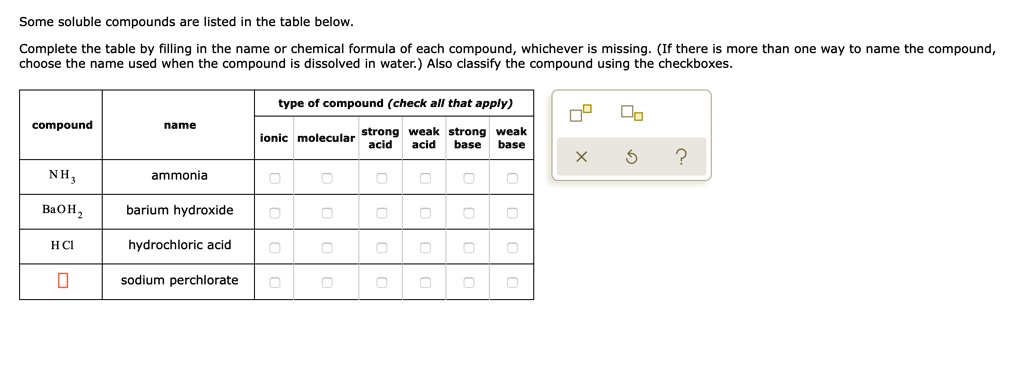
SOLVED: Some soluble compounds are listed in the table below Complete the table by filling in the name or chemical formula of each compound whichever is missing (If there is more than
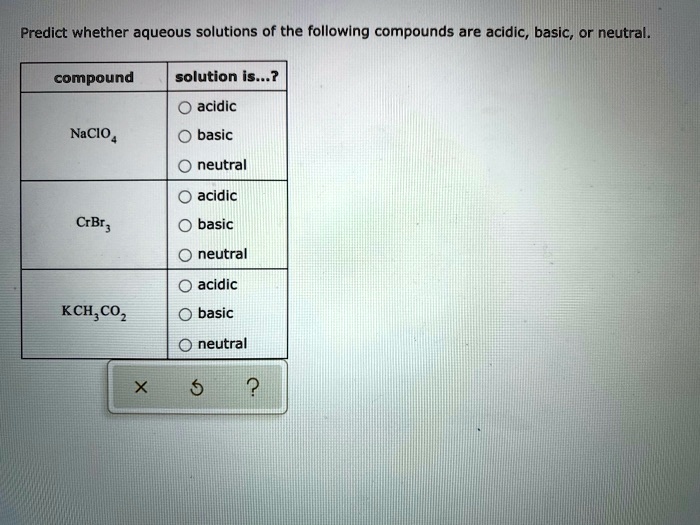
SOLVED: Predict whether aqueous solutions of the following compounds are acidic, basic, Or neutral. compound solution is...? acidic NaCIO basic neutral acidic CrBr basic neutral acidic KCH,COz basic neutral