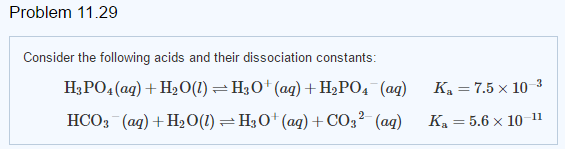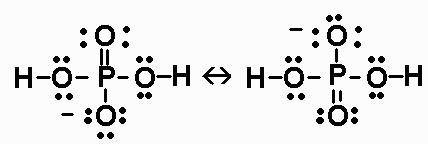Fillable Online UNIT 14 - Acids & Bases ACID BASE HSO4 H3PO4 NO3 H2PO4 ... Fax Email Print - pdfFiller

Acid-Base Test Review Definitions of Acids and Bases 1. Which of the following are Arrhenius acids? a. H2O b. H3PO4 c. NH3 d. H2SO3 2. Which of the. - ppt download
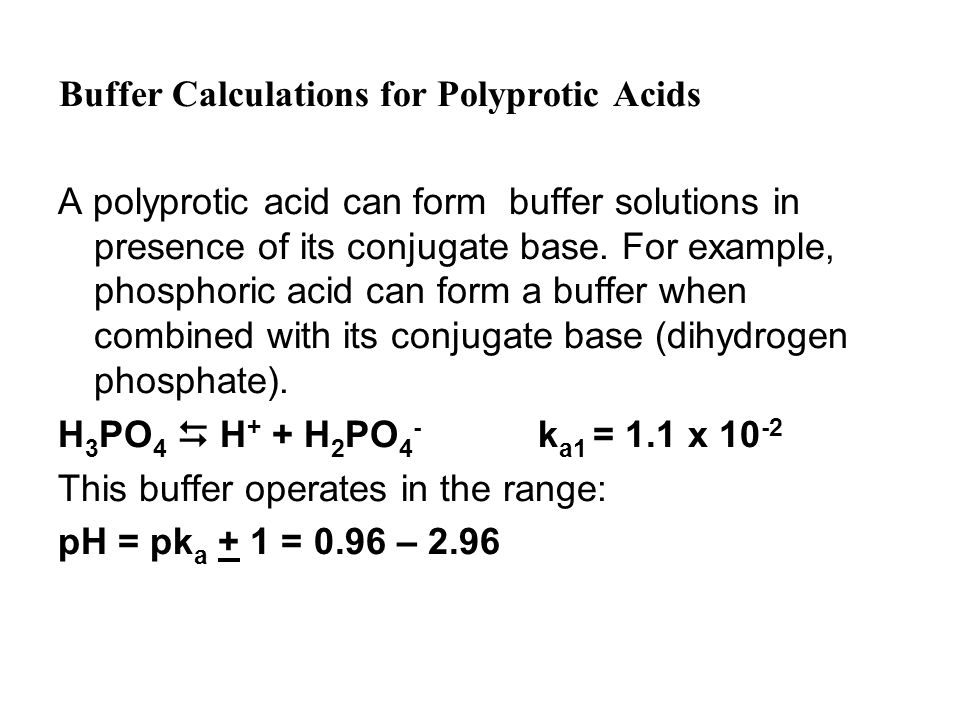
Buffer Calculations for Polyprotic Acids A polyprotic acid can form buffer solutions in presence of its conjugate base. For example, phosphoric acid can. - ppt download
![In the acid base titration [H3PO4(0.1 M)+NaOH(0.1 M)] e.m.f of the solution is measured by coupling this electrodes with suitable reference electrode.When alkali is added pH of solution is in acoodance with In the acid base titration [H3PO4(0.1 M)+NaOH(0.1 M)] e.m.f of the solution is measured by coupling this electrodes with suitable reference electrode.When alkali is added pH of solution is in acoodance with](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/32521666_web.png)
In the acid base titration [H3PO4(0.1 M)+NaOH(0.1 M)] e.m.f of the solution is measured by coupling this electrodes with suitable reference electrode.When alkali is added pH of solution is in acoodance with

Use your understanding of molecular structure to explain why the conjugate bases of acids like formic acid CHOOH, acetic acid CH3COOH, and phosphoric acid are only stable enough to be weak acids;